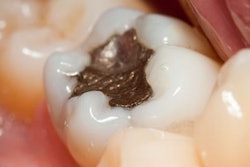
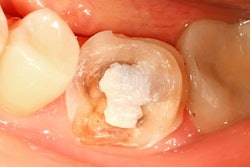
The U.S. Food and Drug Administration (FDA) has developed graphics to go along with its previously announced recommendations regarding the use of dental amalgam in high-risk groups.
Last September, the FDA advised that high-risk individuals, such as pregnant women, avoid getting amalgam fillings "whenever possible and appropriate." Previously, the agency warned that people in these groups may be at greater risk of adverse health events from mercury-containing fillings.
The new graphics illustrate those recommendations and provide information on what patients should know before getting an amalgam filling and who the FDA considers to be high risk. The graphics also outline why the FDA has issued its recommendations and whether existing amalgam fillings should be removed.
 One of the FDA graphics illustrating its new amalgam recommendations. Image courtesy of the FDA.
One of the FDA graphics illustrating its new amalgam recommendations. Image courtesy of the FDA.