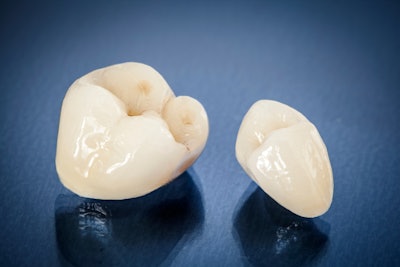
3D dental printing company Formlabs Dental has received 510(k) medical device clearance from the U.S. Food and Drug Administration (FDA) for its Premium Teeth Resin, the company announced in a press release.
The approval governs the company's resin for crowns, inlays, onlays, veneers, and up to seven-unit temporary bridges, according to Formlabs. The resin comprises a nanoceramic biocompatible material that mimics the natural opalescence of teeth and allows users to produce a range of dental appliances using one material. The applications, Formlabs noted, have previously been approved in the European Union.



















