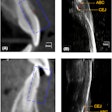
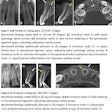
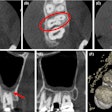
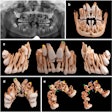
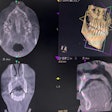
The U.S. Food and Drug Administration (FDA) has created a new Web page designed to provide healthcare providers and the public with the latest information on the health and safety of dental cone-beam CT (CBCT).
The "Dental Cone-beam Computed Tomography" Web page is new to the Radiation-Emitting Products site, according to an FDA spokesperson. The FDA created the page to give healthcare providers, patients, parents, and industry accurate information to help ensure that dental cone-beam CT exams are justified (appropriate) and optimized (exposure settings are selected based on the ALARA -- as low as reasonably achievable -- principle).
"Although the radiation doses from dental CBCT exams are generally lower than other CT exams, dental CBCT exams typically deliver more radiation than conventional dental x-ray exams," the FDA states on the new Web page. "Concerns about radiation exposure are greater for younger patients because they are more sensitive to radiation (i.e., estimates of their lifetime risk for cancer incidence and mortality per unit dose of ionizing radiation are higher) and they have a longer lifetime for ill effects to develop."
Earlier this year, the FDA unveiled a pediatric x-ray imaging Web page and draft guidance to encourage manufacturers of x-ray imaging devices to consider the safety of children in the design of new equipment.
The draft guidance recommends manufacturers design new x-ray imaging devices with protocols and instructions that specifically address their use on pediatric patients.
It also proposes that manufacturers who do not adequately demonstrate that their new x-ray imaging devices are safe and effective in pediatric patients should include a label on their device that cautions against use in pediatric populations.
In addition, several dental groups have issued their own guidelines for appropriate cone-beam CT use. In this month's Journal of the American Dental Association, for example, the ADA's Council on Scientific Affairs released recommendations for the safe use of cone-beam CT in a dental practice, with particular emphasis on patient safety, practitioner training, radiation protection, and dose considerations.
"Since cone-beam CT devices were introduced commercially in the U.S. in 2001, dentists have come to use the technology in increasing numbers," the council wrote. "Yet, although cone-beam CT technologies have advanced rapidly across time, concerns have been expressed about whether the information acquired with cone-beam CT imaging warrants the additional exposure risk, as well as about the level of training, education, and experience required to interpret the cone-beam CT dataset."
In general the ADA and the FDA recommend that clinicians perform dental x-ray examinations, including dental cone-beam CT, only when necessary for the diagnosis or treatment of disease.
The American Academy of Oral and Maxillofacial Radiology, the International Congress of Oral Implantologists, and the American Association of Endodontists also have released position statements and professional guidelines for cone-beam CT use in dentistry.