As digital imaging gradually supplants film, new ways of fine-tuning and improving the technology are surfacing. Dental artifacts can impact the effectiveness of positron emission tomography and magnetic resonance imaging (PET/MRI), but a new method could be part of a solution.
Researchers at Rigshospitalet, Copenhagen University Hospital in Denmark are behind the new method, which uses attenuation correction. Attenuation correction (AC) in PET can ignore dental artifacts, but "metal implant-induced susceptibility artifacts and subsequent signal voids challenge MR-based AC," a problem that has been acknowledged in some studies but has yet to be solved, the researchers noted in their paper published in Proceedings of SPIE (March 21, 2014, Vol. 9034).
"The PET/MR scanner came out late 2011, so it is very new, but has a number of places where it can be improved," stated lead author Claes Ladefoged, MSc, a research assistant at the hospital who has a background in computers science and is working toward his doctorate, in an email to DrBicuspid.com. "In dentistry, we see that more than 40% of the patients have a dental artifact."
Ladefoged and his fellow researchers have proposed a new, clinically feasible method that could potentially solve the issue of dental artifacts. The method combines active shape models (ASM) and the k-NEAREST-Neighbors (kNN) algorithm search to locate and correct dental artifacts that appear on the image of the tooth's surface.
“It performs very well on real patients with dental artifacts.”
"This method is very robust since it can find the landmarks in images that do not include all landmarks, e.g., when the point under the chin or the commissure points are missing, or when the patient has a large dental artifact that results in loss of signal in the T1w [T1-weighted] image," the researchers wrote. The unique method also "performs very well on real patients with dental artifacts, where it was able to find and correct the artifacts."
Conceptually, the method simulates the decision-making process made by an expert, whereby landmarks are used to determine the nature of a signal void, whether it is a hole or dental artifact. In fact, Ladefoged's solution stems from his own process for finding artifacts. "I always view the patient in a sagittal orientation (from the side) so I can see how the hole is positioned in respect to the nose, the esophagus, and the sinuses," he explained. "This method simulates this by simply representing the dental area with an offset to nine landmarks in the head. Furthermore, I use a very simple classifier, kNN, to train a model that can tell me what areas are usually artifact areas and what are normal air regions (sinuses, etc)."
First, the researchers built a point distribution model (PDM) containing the shape of the landmarks along with a local appearance model based on each point. Next, an ASM determines the location of these landmarks in new images based on the PDM's parameters and local appearance information. Lastly, a simple kNN search, which uses a subset of the training set the researchers used to build the PDM, classifies the pixels of the holes as an artifact or actual hole.
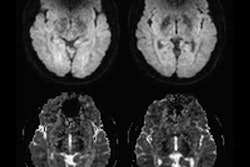
To test the method, they selected 18 patients that had metallic dental implants and implant-induced signal voids. All were part of the hospital's existing PET/MRI referral base of patients with neurological disorders and were selected retrospectively. Of these patients, six had minor artifacts of less than 50-mL signal void volume, eight had major artifacts between 100 mL and 200 mL, and four had extreme artifacts of more than 200 mL. The clinicians used the patients' T1-weighted MR images taken during head/neck and brain protocols, because these images display detailed anatomical structure. And the center slice of these sagittal T1-weighted images were selected because they encompass the largest section of the dental area possible. Additionally, with sagittal orientation, signal voids can be classified using only x and y coordinates.
When testing the method, the artifact region in each of the 18 patients was manually masked and then compared with the filled region resulting after correction. The correction method filled all the artifacts in every patient. In two patients, however, a border line separating the sinus from the artifact was drawn differently, resulting in an overall precision score of 0.958 and a recall score of 1, the researchers noted.
The study also tested 14 patients who did not have dental implants, finding that the method left volumes unchanged in all but two patients. In those cases, the correction overfilled a small amount (0.4 mL) in the bottom part of the right maxillary sinus. The patients had a sinus located lower than is ordinary, "meaning it reached into an area where only artifacts are normally present," the researchers explained.
Ladefoged sees potential for the method to improve the functionality of PET/MR devices that have shortcomings when metal is present. "A much larger area than the metal itself can be affected by signal loss, and therefore a very large area (the entire front of the head) can be incorrectly set to air, causing an almost complete signal loss in the front of the head, potentially hiding a tumor in the mouth," he noted.
Whether clinicians and manufacturers share his opinion about the method's usefulness remains to be seen. "I hope that my method brings us closer to a solution the vendors would like to implement," he wrote. "While PET/MR has yet to find a specific area in dentistry in which it performs better than the alternatives, it is an attractive system due to the low radiation dose (MR compared with CT)."
For now, Ladefoged will make an effort to improve on his method. "Our future work is to train the models on more patients, and also to look at more MR images to see if the model can benefit from more knowledge than simply the offset to landmarks," he wrote.