An international team of researchers has developed new classification criteria for the autoimmune condition Sjögren's syndrome, according to a study in Arthritis Care & Research (April 2012, Vol. 64:4, pp. 475-487).
The classification criteria are the first for Sjögren's syndrome to be based solely on objective clinical tests, noted the Sjögren's International Collaborative Clinical Alliance (SICCA) in a press release issued by the National Institute of Dental and Craniofacial Research (NIDCR), which helped fund the research.
Other criteria historically have permitted various testing subjectivity to enable the classification of this notoriously complex syndrome that affects multiple parts of the body, typically the eyes, salivary glands, and joints. The subjectivity has made standardization of clinical trial enrollment something of a moving target, limiting comparability of research data across studies and impeding the needed robust clinical evaluation of possible new treatments.
Importantly, the American College of Rheumatology (ACR) voted to accept the SICCA criteria. This marks the first time that ACR has approved classification criteria for Sjögren's syndrome and, importantly, recognized the need for rheumatologists, ophthalmologists, and oral medicine practitioners to collaborate in research studies to diagnose the condition.
"The next step will be to present our criteria to representatives of the European League Against Rheumatism, the European equivalent of ACR, with a goal of achieving a single international standard," said Caroline Shiboski, DDS, MPH, PhD, professor at the University of California San Francisco (UCSF) School of Dentistry, and co-lead author on the study.
Three objective tests
Founded in 2003, the SICCA alliance is an integrated research network that spans Argentina, China, Denmark, India, Japan, U.K., and U.S. By sharing their scientific resources, the researchers assembled the first international Sjögren's syndrome patient registry, a major step forward in studying this condition.
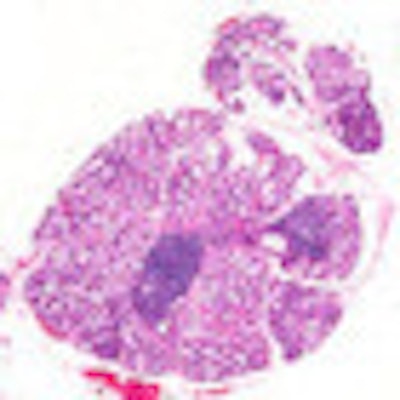
 Photomicrograph of a salivary gland with sites of inflammation indicative of Sjögren's syndrome. Image courtesy of NIDCR.
Photomicrograph of a salivary gland with sites of inflammation indicative of Sjögren's syndrome. Image courtesy of NIDCR.
In the SICCA study, an international expert panel of ophthalmologists, rheumatologists, and oral medicine/pathology specialists began by compiling a consensus list of existing diagnostic tests to assess the various components of Sjögren's syndrome (oral, ocular, and systemic).
Using the various listed tests, the researchers performed comprehensive clinical evaluations on nearly 1,400 participants enrolled in the SICCA Registry. All had possible signs and/or symptoms of Sjögren's syndrome, typical of patients seen in a clinical practice, and were drawn from ethnically diverse patient populations worldwide.
Combining the expert consensus and data collected from study participants, the SICCA scientists developed a set of preliminary classification criteria for Sjögren's syndrome. These criteria were then validated using novel statistical methods and comparisons with existing classification approaches, most notably the American-European Consensus Group (AECG) criteria. Published in 2002, the AECG criteria are currently the most frequently cited in Sjögren's syndrome studies.
The new SICCA classification criteria stipulate that to be classified with Sjögren's syndrome, research participants must be positive for at least two of three objective diagnostic tests:
- Blood test: Positive serum levels of the SSA and/or SSB antibody or positive serum levels of the rheumatoid factor antibody and elevated antinuclear antibody titers. All antibodies are associated with the syndrome.
- Ocular surface staining: Measures the dissipation rate of a specialized dye that is applied to the tear film that bathes the surface of the eye. A score of three or more is considered to be positive.
- Salivary gland biopsy: A pathologist examines the biopsy for sites of inflammation. One or more sites of inflammation per 4 mm squared area is considered positive.
"Uniform classification criteria based on objective tests are important to help ensure that participants in clinical studies and treatment trials on Sjögren's syndrome are correctly classified as having the disease," said Dr. Shiboski. "This is especially important with respect to treatment trials to avoid exposing individuals to new investigational drugs and any potential toxicity if they don't have Sjögren's syndrome."
The SICCA study also reinforced that the subjective may not always be as informative as it at first seems.
"Dry mouth and eyes long have been associated with Sjögren's syndrome," said Dr. Troy Daniels, DDS, professor in the UCSF School of Dentistry and a senior author on the paper. "But our data showed that although patients may complain of dry mouth and eyes, these symptoms lack a statistically significant association with positive antibodies, ocular staining, and/or salivary biopsy. This shows the tremendous complexity of Sjögren's syndrome and the true value of assembling more objective classification criteria moving forward."