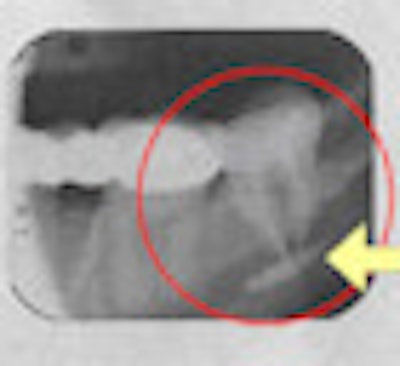
The FDA, ADA, and AAE do not condone it, nor do U.S. dental schools. Dozens of lawsuits have been filed by patients -- most of them settled out of court -- claiming permanent jaw damage and other debilitating side effects following its use. So why do dentists continue to use Sargenti paste for endodontic obturation?
Developed in the 1940s and 1950s by Angelo Sargenti, D.M.D., a Swiss dentist who died in 1999, and introduced in the U.S. at the 1958 ADA meeting, the Sargenti technique gained popularity in the 1970s. But it just as quickly became the object of complaints about its side effects, with patients claiming they suffered permanent jaw damage and developed osteomyelitis following root canal procedures involving Sargenti.
The issue is Sargenti's key ingredient: paraformaldehyde. The American Association of Endodontists (AAE) recommends against its use, noting that "paraformaldehyde-containing endodontic filling materials or sealers (frequently known as Sargenti pastes, N2, N2 Universal, RC-2B, or RC-2B White) should not be used for endodontic treatment because those materials have proven to be both unsafe and ineffective" and that its use falls "below the standard of care." Sargenti paste contains 6.5% paraformaldehyde.
“Dentists who use it recklessly expose patients to something that is clearly harmful.”
— Stephen Cohen, D.D.S., M.S.
"Extensive scientific research has proven unequivocally that paraformaldehyde-containing materials can cause irreversible damage to tissues near the root canal system including the following: destruction of connective tissue and bone; intractable pain; paresthesia and dysesthesia of the mandibular and maxillary nerves; and chronic infections of the maxillary sinus," the AAE notes. Scientific evidence has also revealed that active ingredients in the material have been shown to infiltrate the blood, lymph nodes, adrenal glands, kidney, spleen, liver, and brain, according to the organization.
"Public health concerns and litigation have made the AAE aware of a significant number of patients who have suffered injuries as a result of treatment with paraformaldehyde-containing filling materials and sealers," the AAE's position statement on this material states. "Undoubtedly, there are many other patients who have also suffered injuries because of these materials, but whose injuries have not been publicly disclosed."
The ADA and all U.S. dental schools also advise against the use of Sargenti paste. Like most professional dental organizations that consider it "below the standard of care," the Florida Board of Dentistry says Sargenti does not "meet the minimum standards of performance for competent dental practice," and "specifically finds that Sargenti... can cause severe and irreversible damage to patients." Outside the U.S., it is not licensed for sale in Canada, and Belgium, France, the Netherlands, and Japan have formally banned formaldehyde.
But some contend the issue is more about economics than public safety.
"It works. It is less painful to patients having root canals, and patients save their teeth with less cost," said Alvin Arzt, D.D.S., a retired Pennsylvania dentist who is the founder and past president of the American Endodontic Society (AES); president of N2 Products, a company that could sell Sargenti paste if the FDA allowed it; and one of the most vocal proponents of Sargenti. "And it is done by their general dentist, rather than having to wait and go to the limited practitioner of endodontics [who] doesn't want general dentists to do successful root canals because they [endodontists] can charge $1,000 for them."
Why still legal?
In 1993 an FDA dental advisory panel confirmed that the safety and effectiveness of N2 -- which is classified as a medical device -- were unproven, and FDA regulations prohibit the interstate shipment of unapproved drugs or individual components used to compound them. Mail order shipments of Sargenti from out-of-state pharmacies in quantities greater than for single-patient use (5 grams) are considered a bulk sales order rather than a prescription, thus violating FDA regulations.
To date, the agency has still not granted marketing clearance to N2, so no company is allowed to sell it in the U.S. The FDA considers other root canal fillers, such as gutta-percha, Class I medical devices because the material does not stay in the body; these products do have FDA marketing clearance.
However, because dentists are not regulated by the FDA, they can circumvent these rules and order Sargenti paste from compounding pharmacies. And the FDA is notoriously lax in its regulation of these pharmacies, according to Stephen Cohen, D.D.S., M.S., former chief of the endodontics department at the University of the Pacific Arthur A. Dugoni School of Dentistry and the author of several endodontic textbooks. Although occasionally, following high-profile court cases, the FDA will sanction pharmacies that get caught selling more than 5 grams.
"The FDA has been derelict in enforcement of rules that already exist," he said in an interview with DrBicuspid.com. "When something gets into press, they will suddenly wake up and shut down a few labs in Arizona and California."
Dr. Cohen has testified before congressional hearings numerous times about the dangers of Sargenti paste. In the 1980s, he testified "every few weeks" in court cases stemming from patients' injuries from Sargenti, he said. One of those involved a 38-year-old Florida nurse who had to have her entire jaw replaced and received a $1 million settlement from the dentist who performed the procedure.
"Dentists who use it recklessly expose patients to something that is clearly harmful," Dr. Cohen said. "There's no way a dentist can say, 'I didn't know,' because there's more than a significant amount of scientific evidence to support its harmful effects."
Dr. Cohen, who has also treated patients who developed problems following Sargenti root canals, explained that the inferior alveolar neurovascular bundle often gets damaged after exposure to the material.
"Unless it's treated quickly, surgically, within a few days, the damage is permanent," he said. "In the majority of cases, there's nothing I can do."
Dr. Cohen estimates that more than 10,000 patients have sustained injuries from Sargenti. And he's at a loss as to why dentists would continue to use it in the face of "overwhelming evidence" about its dangers.
"I am in disbelief that anyone would use it," Dr. Cohen said, noting that the "vast majority of dentists are smart enough to avoid it," and that most use conventional obturation materials such as gutta-percha.
Hogwash or a time bomb?
Even so, thousands of dentists reportedly continue to use it, although most shy away from publicly acknowledging it. Mark Piacine, D.D.S., a general practitioner in Pottstown, PA, and president of the AES, which advocates its use, estimates that he's used Sargenti in more than 4,000 root canals since 1973 with no problems.
In an interview with DrBicuspid.com, he called the criticism and warnings about its dangers "just hogwash, nonsense."
“There are no peer-reviewed scientific studies that show Sargenti is more dangerous or less successful than any other sealer.”
— Patrick Wahl, D.M.D., M.B.A.
Dr. Piacine believes the controversy boils down to a turf war between general dentists and endodontists who are upset about losing lucrative root canal business to the generalists, citing a 1975 AAE meeting in which he says they discussed efforts to stop Sargenti's success. "They won't admit it, but it's a turf war," he said.
Regarding the toxicity of paraformaldehyde, it's a matter of dosage, Dr. Piacine said. "We're only putting 4% in the tooth, and it doesn't go anywhere unless you're using bad techniques to push it beyond the apex," he said. "If you misuse any material, you can have problems."
Most new dentists don't use it because they're afraid of getting sued, Dr. Piacine said. But if patients ask, Dr. Piacine tells them he's using Sargenti.
Edwin Zinman, D.D.S., J.D., a San Francisco attorney specializing in dental malpractice, has handled dozens of lawsuits involving Sargenti injuries, some resulting in punitive damages against pharmacies. He also faults the FDA for not enforcing its own regulations forbidding interstate shipments of Sargenti ingredients and bulk sales by pharmacies.
"It's cheap and quick and excuses poor endodontic techniques because it's such a potent chemical," Dr. Zinman told DrBicuspid.com. He called Sargenti a "chemical time bomb that can explode catastrophically if it gets past the root tip."
The "small but dangerous number of dentists" still using it conceal it or falsify their records, Dr. Zinman said. "They know that if an attorney finds out, it's like putting a red flag in front of a bull," he said.
Dr. Artz echoed Dr. Piacine's opinion that the issue reflects a turf war between general practitioners and endodontists who don't want to lose root canal business, calling the negative assessments of Sargenti "false, lies and distorted research and reports, put together by the AAE."
Dr. Artz believes poor dentistry, not Sargenti paste, was to blame in the cases in which patients sustained injury. "You have to do a thorough job of obturation, cleaning and shaping the canal, before using N2," he said. Improper sealing can also result in tooth failure, he added.
"I did 11,000 N2 root canals and never had a lawsuit," Dr. Arzt said. "My wife had five, and I had three myself."
The conventional disinfectant for root canals, sodium hypochlorite (Clorox), still leaves behind 30% of the bacteria, he asserted, while N2 achieves greater disinfection by killing all but 4% of the bacteria, Dr. Arzt claimed. And when N2 is mixed with powder and liquid for application, the paraformaldehyde is diluted to 4% levels, he added.
In addition, conventional root canals have only an 80% success rate, compared with N2's 97% success rate, according to Dr. Arzt. "Why would you do something that only has an 80% success rate when you can use N2 without pain?" he asked.
He estimates that 30,000 dentists worldwide are still using Sargenti. But they may not admit it "because they're afraid they'll get sued," he said. "There's a lot of propaganda about it."
No peer-reviewed studies
Another Sargenti advocate is Patrick Wahl, D.M.D., M.B.A., an endodontist in Wilmington, DE. Although he doesn't use it and even doubts it's all that beneficial, Dr. Wahl believes the opposition to Sargenti is "primarily a political thing."
"I would like to try Sargenti cement, but I'm concerned about the unfounded hysteria surrounding it and the endodontists and hired guns who are eager to profit from frivolous lawsuits," Dr. Wahl told DrBicuspid.com. "There are no peer-reviewed scientific studies that show Sargenti is more dangerous or less successful than any other sealer."
Cleaning and shaping the canal is more important than the sealer, he said. "It's outrageous that the FDA hasn't approved it," he said.
Dr. Wahl called the injury cases "very unfortunate," but says there's no evidence to prove they were caused by Sargenti. "They could have been caused by all kinds of things, like overtreatment," he said. "And some could be made worse by aggressive surgeries."
If he were to use it, he's convinced it would work just as well as any other sealer, he added. "Millions of teeth have been saved by Sargenti cement, and the incredibly low number of adverse events that have been reported in any way is compelling evidence of the material's safety," he said.
But Louis Rossman, D.M.D., a professor of endodontics at the University of Pennsylvania School of Dental Medicine who maintains a endodontic practice in Philadelphia, disagrees.
"Research demonstrates using formaldehyde material inside a tooth is not healthy for surrounding tissues," he told DrBicuspid.com. "You can set a standard of care, but it's up to the practitioner to ethically follow that. I think it comes down to education, but as long as people will look for a shortcut that is more profitable, the people who will lose are the patients."
Gordon Christensen, D.D.S., M.S.D., Ph.D., believes the AAE and AES need to work together to settle this debate once and for all.
"About 20 years ago, when the Sargenti technique was overtly criticized by some in the U.S. endodontic community, I looked into the concept during many of my international speaking events and in the international literature," he told DrBicuspid.com. "I found at that time the Sargenti technique was used with apparent success throughout much of the world. I sent letters to the AES and to the AAE [and] suggested that the two groups should get together to plan and fund a clinical and basic science study to compare the two concepts."
To the best of his knowledge, that study has yet to be conducted.
"I still suggest such a study needs to be done to prove or disprove in a scientific manner the many allegations and heated discussions concerning the Sargenti concept. Until the two groups can talk to one another, the emotional and raging discussions remain just that!"
Copyright © 2010 DrBicuspid.com