Root canals can rob teeth of their natural biological response when infected tissue is replaced with synthetic biomaterials. To combat this problem, researchers have developed a method -- inspired by 3D printing technology -- for engineering prevascularized dental pulp that could be used in root canals of the future.
Their results could form the basis for a strategy for creating pulp that one day could be used for routine dentistry, they report in the journal Scientific Reports (June 12, 2017). The 3D printing-inspired process that they used is based on their previous work fabricating artificial capillaries.
"This result proves that fabrication of artificial blood vessels can be a highly effective strategy for fully regenerating the function of teeth," said Luiz Bertassoni, PhD, DDS, one of the study authors. "We believe that this finding may change the way that root canal treatments are done in the future."
Dr. Bertassoni is an assistant professor of restorative dentistry at Oregon Health and Science University (OHSU) School of Dentistry in Portland and an honorary bioengineering lecturer at the University of Sydney dental school in Australia. His collaborators on this research were from the OHSU School of Dentistry, the department of biomedical engineering at the OHSU School of Medicine, and the University of Birmingham School of Dentistry in the U.K.
Talking 'bout regeneration
Dental pulp is highly vascularized, innervated, and unmineralized connective tissue, the authors noted. However, current root canal treatment typically involves the removal of infected or necrotic tissue and replacement with inert synthetic materials, thereby sacrificing the tooth's biological response, they wrote.
Regeneration of pulp tissue to restore tooth function, known as regenerative endodontics, could offer an alternative to traditional root canal treatment, but this requires novel strategies for regenerating vascularized pulp, according to research in the field. Previous researchers have reported on the regeneration of vascularized pulp by culturing endothelial or stem cells, although such strategies require time-intensive biological processes.
“This finding may change the way that root canal treatments are done in the future.”
A more simplified biofabrication strategy compatible with short-term vasculature formation that has greater clinical potential is lacking, according to the authors of the current study. Use of an engineered vasculature present from the onset of the regenerative process would be a better strategy for preventing hypoxic conditions in tissue prior to neovasculogenesis, they wrote. To that end, they investigated a novel strategy they developed for fabricating prevascularized pulp-like hydrogel tissue constructs in full-length root canals in vitro.
First, they evaluated the extracellular microenvironmental conditions, such as microstructure, degradation, and swelling, that enhance the viability, spreading, and proliferation of odontoblast-like cells embedded in methacryloyl (GelMA) hydrogels that they synthesized. The hydrogels, which have been used for various tissue engineering applications but not as much for dental pulp regeneration, had tunable physical and mechanical properties due to varying polymer concentrations.
Their results suggested that odontoblast-like cells encapsulated in hydrogels with higher stiffness had greater spreading, proliferation, and viability. Endothelial colony forming cells seeded on stiffer gels demonstrated greater spreading and tendency to form endothelial monolayers.
For the next phase of their work, the researchers used premolars extracted from people for orthodontic reasons that were sectioned in root fragments 9-mm long with approximately 1.5-mm apical foramen diameter. They performed root canals in the root fragments and made 500-µm diameter microchannels throughout the root canals.
The researchers fabricated prevascularized, full-length, dental pulp-like tissue constructs made of GelMA hydrogel prepolymer and containing odontoblast-like cells that they inserted into the root canals. They placed endothelial cells isolated from the interior lining of blood vessels in these microchannels. Within seven days, dentin-producing cells proliferated near tooth walls, and artificial blood vessels formed inside the teeth.
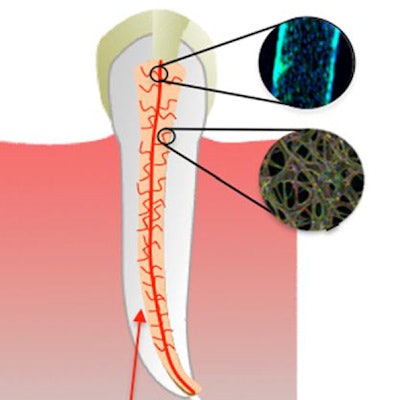
 Schematic diagram illustrating the basic steps of the proposed strategy to engineer prevascularized full-length dental pulp-like tissue constructs. (A) The root canal is prepared following common endodontic procedure using endodontic files. (B) A premade sacrificial fiber is positioned in the root canal. A cell-laden hydrogel is loaded into the canal and photopolymerized. (C) After the hydrogel photopolymerization, the sacrificial fiber is removed, creating a hollow microchannel that traverses the entire length of the canal, from the apex through the pulp chamber. (D) Endothelial cells are seeded in the fabricated microchannel to engineer the core vascular capillary in the dental pulp, thus resulting in a prevascularized full-length dental pulp-like tissue construct. All images courtesy of "A Novel Strategy to Engineer Pre-Vascularized Full-Length Dental Pulp-like Tissue Constructs" (Scientific Reports, June 12, 2017).
Schematic diagram illustrating the basic steps of the proposed strategy to engineer prevascularized full-length dental pulp-like tissue constructs. (A) The root canal is prepared following common endodontic procedure using endodontic files. (B) A premade sacrificial fiber is positioned in the root canal. A cell-laden hydrogel is loaded into the canal and photopolymerized. (C) After the hydrogel photopolymerization, the sacrificial fiber is removed, creating a hollow microchannel that traverses the entire length of the canal, from the apex through the pulp chamber. (D) Endothelial cells are seeded in the fabricated microchannel to engineer the core vascular capillary in the dental pulp, thus resulting in a prevascularized full-length dental pulp-like tissue construct. All images courtesy of "A Novel Strategy to Engineer Pre-Vascularized Full-Length Dental Pulp-like Tissue Constructs" (Scientific Reports, June 12, 2017). Representative images of prevascularized pulp-like tissue construct. (A) Longitudinal and (B) cross-sectional views of GelMA hydrogels loaded with green fluorescent microparticles showing the fabricated microchannel after being perfused with a red fluorescent microparticle solution. The channels cross the entire length of the root. (C,D) Photographs of GelMA hydrogels from longitudinal and occlusal perspectives inside a full-length root fragment. Root fragments were stabilized prior to hydrogel loading and microchannel fabrication, and were separated to retrieve the constructs and illustrate the position of the hydrogel inside the tooth. Microchannels were perfused with red food dye.
Representative images of prevascularized pulp-like tissue construct. (A) Longitudinal and (B) cross-sectional views of GelMA hydrogels loaded with green fluorescent microparticles showing the fabricated microchannel after being perfused with a red fluorescent microparticle solution. The channels cross the entire length of the root. (C,D) Photographs of GelMA hydrogels from longitudinal and occlusal perspectives inside a full-length root fragment. Root fragments were stabilized prior to hydrogel loading and microchannel fabrication, and were separated to retrieve the constructs and illustrate the position of the hydrogel inside the tooth. Microchannels were perfused with red food dye.The authors explained that the rationale behind their proposed strategy was to make sure a functional vascular-like conduit could be formed through the engineered pulp scaffolds from the beginning of the regenerative process. This would ensure the optimization of oxygen and nutrient diffusion and waste removal through the length of the scaffolds during the remodeling process.
"Although we report proof-of-principle experiments utilizing animal cell sources that are not compatible with direct clinical applications and are only intended at initial screening of the developed protocols, we argue that the proposed approach may form the basis for a simple and effective strategy for the engineering of vascularized dental pulp with potentially beneficial translational outcomes," they wrote.
 Cell spreading and monolayer formation by endothelial colony forming cells (ECFCs) on 2D GelMA hydrogels. Representative images of ECFCs on (A,B) 5%, (C,D) 10%, and (E,F) 15% GelMA hydrogels after 1 and 6 days in culture, stained for actin (green) and DAPI (blue) showed increased cell spreading and cell density on stiffer substrates at both early and late time points. (G) Quantification of the number of cells per unit surface area on day 6, representative of cell coverage on the hydrogels and the propensity for endothelial monolayer formation suggests that stiffer GelMA hydrogel substrates support ECFC proliferation and monolayer formation. Statistical significance is represented by * for p < 0.05.
Cell spreading and monolayer formation by endothelial colony forming cells (ECFCs) on 2D GelMA hydrogels. Representative images of ECFCs on (A,B) 5%, (C,D) 10%, and (E,F) 15% GelMA hydrogels after 1 and 6 days in culture, stained for actin (green) and DAPI (blue) showed increased cell spreading and cell density on stiffer substrates at both early and late time points. (G) Quantification of the number of cells per unit surface area on day 6, representative of cell coverage on the hydrogels and the propensity for endothelial monolayer formation suggests that stiffer GelMA hydrogel substrates support ECFC proliferation and monolayer formation. Statistical significance is represented by * for p < 0.05.Intricacies ignored
The authors noted, however, that cells from various animals may not behave in the same ways as human dental pulp cells. Additionally, the current design does not yet duplicate the intricate morphology of pulp microvasculature or other important tissue components, such as nerves, fibroblasts, and undifferentiated stem cells, all of which would be required for complete and effective regeneration of dental pulp, they conceded.
"Despite these obvious limitations, we are convinced that the engineered microchannels could enhance the formation of cell-based microvessels in a hydrogel loaded with co-cultures of endothelial cells and dentin progenitor cells of human source," the authors concluded. "This forms the basis for future studies in our laboratory, as well as a more thorough in vivo analysis of the effectiveness of the proposed approach."