BioMatRx, a Minnesota start-up founded in April 2010 by three brothers -- one an endodontist -- is working to use the inherent regenerative capabilities of the stem cells found at the apex of the root of a tooth to revolutionize endodontic therapy.
The idea for the company and the process struck Todd Geisler, D.D.S., and his brother Graham Geisler as they were returning home from the American Association of Endodontists (AAE) annual meeting in 2009. Dr. Geisler had hosted hands-on workshops on the regenerative techniques for permanent teeth.
Research has demonstrated that deliberate bleeding and clotting could restore blood flow to a damaged tooth and repair its walls and roots, he noted.
"The idea to hone the technique and make it a better option was on the forefront of his mind [following the 2009 AAE meeting]," Graham told DrBicuspid.com. "His talks generated a lot of interest, and endodontists were asking where to get all the supplies for this procedure because it's outside of the current realm of practice. So we came up with a convenience package," the RegendoGel Pak.
“The process could be better than a root canal.”
— Graham Geisler, BioMatRx
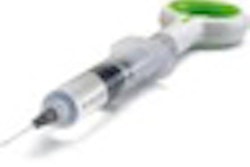
Like a conventional root canal, the BioMatRx approach first requires the endodontist to clean out the diseased root and disinfect the tooth. Next, a low-volume blood draw from the patient's arm is performed, and the blood is placed into a centrifuge where it is spun at a specific speed for a prescribed amount of time. Plasma is extracted and combined with a coagulant in order to give it the consistency of a gel. To further prepare the tooth, bleeding is stimulated in the root to release stem cells into the root canal.
"Then we inject the gel into the tooth to react with the stem cells," explained Jamison Geisler, BioMatRx's chief marketing officer. "Basically, in order to get the tissue regeneration to happen, you need three elements. One is the stem cells already present in the root. We provide the other two: the scaffold and growth factors."
Clinical trials needed
Key innovations have helped improve the method that BioMatRx is offering with its product. In current regenerative methods, a blood clot is put in the root of the tooth and then it is restored. Dr. Geisler wanted to remove red and white blood cells from the injection into the tooth, both of which release enzymes that are harmful to the scaffold when they die out, so he incorporated the use of a centrifuge to separate them into the process.
"The process could be better than a root canal," said Graham. "When successful, you now have a living tooth that is reconnected to the immune system and can fend off bacteria. It promotes a stronger root that matures, thickens, and lengthens."
But how much of an improvement the process is over a traditional root canal is still unclear, the Geislers admit.
"We can't say for sure because there are no published, prospective, randomized, double-blind human clinical trials," said Jamison. But there are more than 120 case studies in the AAE database (a database of case studies submitted by practitioners hoping to identify the most predictable regeneration protocols), he noted. Results, such as the maturing of the root and revascularization, can take up to one to two years to fully manifest, he noted.
Available next year
As BioMatRx gears up to introduce this new technique to the market, one of the company's primary goals is to minimize potential adoption issues among endodontists.
"Obviously this is a new procedure, but the industry is very adamant about going forward and pursuing the regeneration or tissue engineering arena," said Graham. To enhance its appeal, the Geislers are encouraging endodontists to rely on their assistants.
"A barrier to adoption that I noticed during workshops was that otherwise enthusiastic endodontists would say, 'I don't want to learn phlebotomy,' " said Graham. "But this is something that could be shifted to an assistant instead of the endodontist taking time to learn or do that actual procedure. It can be done while the endodontist is cleaning out the tooth and disinfecting it. The gel cone is presented to him in a similar way to how the rubber is used."
When asked who BioMatRx considers as its competition, Graham responded, "We've been trying to figure that out. There are other companies that could easily drop into it, but we do have a strong intellectual property license." Other companies that are developing or providing stem cell products and services for dentistry include Odontis, Bioeden, and StemSave.
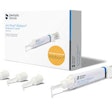
BioMatRx's first product, the RegendoGel Pak, will sell for $199 and does not require does not require FDA clearance because everything in it already has FDA approval or clearance, Jamison said. The Regendo System, which contain a custom centrifuge and five RegendoGel Paks, will sell for $1,500. Commercial launch is tentatively set for April 2011. BioMatRx is also looking to solidify contacts with distributors such as Patterson Dental and Henry Schein and has been asked to present at AAE's next annual session, according to Graham.
"It will be a great platform to teach the procedure and have direct contact with our target market," he said. "It does give us a competitive advantage."
Copyright © 2010 DrBicuspid.com