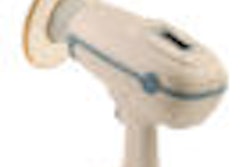
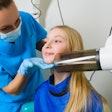
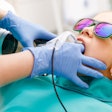
The KaVo Group has acquired Aribex, developer and manufacturer of the Nomad handheld, portable x-ray system.
Details of the transaction were not disclosed, but the company will remain at its headquarters in Orem, UT, with the same management and operational staff, according to D. Clark Turner, PhD, who founded the company in 2003 and is the former chairman and CEO. The company currently has 58 employees, he noted.
Aribex serves the dental, veterinary, security, and industrial inspection segments and gained U.S. Food and Drug Administration clearance for its flagship product, the Nomad, in July 2005. A second version, the Nomad Pro, was released in 2008. Today the Nomad Pro sells for $7,495, while the Classic sells for $6,495, according to Turner.
The KaVo Group, part of Danaher, comprises a number of brands in the dental market, including KaVo, Gendex, DEXIS, i-CAT, Instrumentarium, Soredex, Pelton & Crane, and Marus.
Following the acquisition, both the Aribex name and Nomad brand will be retained and the company will continue to have its own presence in the marketplace and at tradeshows. Aribex will also keep its existing U.S. sales channels but believes the Danaher presence internationally will allow the company to grow its international sales beyond what it could have done on its own, Turner added.
"Danaher likes to maintain the brand names and visibility of its associated companies, and they have a good reputation for allowing their operating companies to have a lot of autonomy," he said. "Most people who are associated with Aribex in any way won't see any difference in terms of operations and people."
Turner, who is currently CTO and head of R&D for Aribex, plans to remain with the company to help with the transition and to work on some new products in its pipeline, although he admits he also has some research interests outside of the dental field that he hopes to pursue in the long term.
Radiation exposure studies
Since its founding, Aribex has paved the way for the Nomad and other mobile x-ray devices to come to market in the U.S. by gaining the necessary state regulatory approvals. Only two states have still not approved the Nomad to be used as a handheld device: New Hampshire and Delaware, according to Turner.
Aribex has long maintained that its products differ from its competitors with regard to radiation exposure because of five unique features:
- Proprietary shielding around the x-ray tube to minimize leakage radiation
- A built-in leaded acrylic shield to protect the operator from backscatter
- A lead-shielded positioning indicating device (PID) or cone
- A 6-cm-diameter PID, which reduces the area irradiated to 56.2% of that for conventional x-ray units with 8-cm-diameter PIDs
- A 0.4-mm x-ray focal spot compared with a 0.6-mm focal spot for conventional units
The company has conducted and published numerous studies demonstrating the safety and efficacy of the Nomad, both for patients and practitioners. Radiation studies conducted by Aribex have found that in a "worst case" situation -- taking 60 exposures per hour at a maximum exposure of 0.99 seconds each -- an operator has the potential to receive 3.6 mR/hr leakage at the hand. The maximum allowable radiation leakage, per FDA regulations, is 100 mR/hr.
Independent research also has found the Nomad to be safe. A 2008 study in Dentomaxillofacial Radiology (February 2008, Vol. 37:2, pp. 109-112), for example, concluded that "the Nomad presents risks that are no greater than with standard dental radiography units to the patient or operator, and the measured doses are well below recommended levels."
Similarly, a study in the March 2009 Journal of Forensic Sciences (Vol. 54:2, pp. 415-421) found that operator exposure to backscatter radiation while using the Nomad was well below established occupation exposure limits.
And in a study in the September 2010 Forensic Science International (Vol. 201:1-3, pp. 112-117) that compared the Nomad with one wall-mounted system and two other mobile devices, researchers from Katholieke Universiteit Leuven concluded that "occupational dosimetry showed doses at the operator's hand being lowest with protective shielding (Nomad: 0.1 microGy)."
Aribex has also worked hard to protect its intellectual property and in 2009 sued several companies with competing products over alleged patent infringement. That lawsuit was settled in 2010, with the companies named in the lawsuit agreeing to stop marketing their handheld x-ray devices in the U.S. However, with new competitors coming into the U.S. market, Turner said there may be some new patent lawsuits in the offing.
"We are likely to be re-engaging with patent litigation, and with Danaher behind us, it should help us significantly with our patent enforcement strategy," he said.