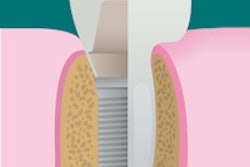
RegeneCure has begun a clinical study using the company's proprietary ammonio methacrylate copolymer type A (AMCA) guided bone regeneration (GBR) dental membrane for patients requiring dental implants.
The AMCA GBR membrane is entirely synthetic, eliminating the risk of contamination by pathogens present in animal-tissue-derived membranes, according to the company. It is also strong and degrades slowly over time, giving the natural bone more time to properly regenerate. In addition, the membrane accelerates healing by enabling cell adherence, proliferation, and differentiation of stem cells into the bone tissue, while preventing connective tissue from infiltrating into the healing space.
A total of 32 patients suffering from insufficient bone volume for dental implant placement will be enrolled in the prospective, randomized, controlled study. One study arm, comprising 16 patients, will use RegeneCure's membrane; the other study arm, also comprising 16 patients, will use a collagen membrane.
Study participants will include nonsmokers and smokers, in order to provide clinical data for potential use in both smoking and nonsmoking populations.
The study will examine if RegeneCure's membrane enables a similar or better amount of lateral bone fill compared with the collagen membrane, which is considered the gold standard treatment today. Each patient will be followed during a six-month period.