More than 80% of patients with head and neck cancer develop painful oral mucositis during radiation treatment. Researchers from the Mayo Clinic and other institutions compared two topical therapies (mouthwashes) with a placebo mouthwash to measure the pain relief they provided.
Patients who used either of the mouthwashes experienced significantly reduced oral mucositis pain compared with patients who used the placebo mouthwash, they reported in the Journal of the American Medical Association (April 16, 2019). The study authors did caution that their results fell short of an essential clinical improvement measurement.
"Among patients undergoing head and neck radiotherapy, the use of doxepin mouthwash or diphenhydramine-lidocaine-antacid mouthwash compared with placebo significantly reduced oral mucositis pain during the first four hours after administration," wrote the authors, led by Terence Sio, MD, of the department of radiation oncology at the Mayo Clinic Hospital in Phoenix.
Significant complication
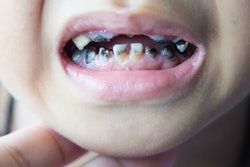
Oral mucositis is a significant complication of cancer treatment. The painful mouth sores can affect a patient's ability to eat and drink and also increase the risk of systemic infections. Few interventions have proved able to reduce their occurrence or the pain, the authors noted.
The researchers wanted to measure the pain relief provided by two types of mouthwashes in a randomized, controlled trial of 275 patients with head and neck cancer (almost 80% of patients were men) undergoing radiation therapy. All the patients had an oral mucositis pain score of 4 points or greater (scale 0 to 10) and were followed for a maximum of 28 days. Patients were told to rinse with the mouthwash for one minute then spit it out. Almost 230 patients completed the treatment.
The patients were randomly assigned to one of three groups:
- The doxepin mouthwash (25 mg/5 mL water) group included 92 patients.
- The diphenhydramine-lidocaine-antacid group included 91 patients.
- The placebo group included 92 patients.
The diphenhydramine-lidocaine-antacid mouthwash (5.1-mL total solution) was composed of 1.7 mL each of diphenhydramine (12.5 mg in a 5-mL alcohol-free solution), lidocaine (2% viscous solution), and an antacid (200 mg of aluminum hydroxide, 200 mg of magnesium hydroxide, and 20 mg of simethicone in a 355-mL solution).
Mucositis pain during the first four hours decreased in each of the groups:
- The pain decreased by 11.6 points in the doxepin mouthwash group.
- The pain decreased by 11.7 points in the diphenhydramine-lidocaine-antacid mouthwash group.
- The pain decreased by 8.7 points in the placebo group.
The between-group difference was 2.9 points (95% confidence interval [CI], 0.2-6.0; p = 0.02) for the doxepin mouthwash versus the placebo and 3.0 points (95% CI, 0.1-5.9; p = 0.004) for the diphenhydramine-lidocaine-antacid mouthwash versus the placebo.
The researchers also found that patients in the doxepin mouthwash group noted more drowsiness compared with the placebo (by 1.5 points [95% CI, 0-4.0]; p = 0.03). The patients also reported more unpleasant taste (by 1.5 points [95% CI, 0-3.0]; p = 0.002) and stinging or burning (by 4.0 points [95% CI, 2.5-5.0]; p < 0.001).
Fatigue was reported by five patients in the doxepin mouthwash group but by no patients in the diphenhydramine-lidocaine-antacid mouthwash group.
Beneficial effect
The authors listed three possible limitations of their study:
- The trial was not designed to compare the mouthwashes directly or to evaluate the combined analgesic effect of both preparations together.
- Other factors such as chemotherapy choice, size of the radiotherapy field, and primary site (oral cavity or oropharynx) could have affected the severity of mucositis pain.
- The study was limited to assessing pain control for only up to four hours.
“This suggests that a subgroup of patients may have experienced meaningful benefits from the diphenhydramine-lidocaine-antacid mouthwash.”
The researchers cautioned that, despite the pain reductions measured, the mean differences in pain reduction fell short in an important measurement.
"The mean differences in pain reduction by the area under the curve for both treatment intervention mouthwashes were less than the minimal clinically important difference of 3.5 points," the authors wrote.
However, this study provides useful clinical results, according to the authors of an editorial accompanying the published study.
While the results did not meet the minimal clinically important difference threshold mentioned above, practitioners may consider using the doxepin mouthwash as a supplemental analgesic to reduce the number of systemic opioids needed for pain relief, wrote Sharon Elad, DMD, of the division of oral medicine at the Eastman Institute for Oral Health at the University of Rochester Medical Center in New York, and Noam Yarom, DMD, of the Tel Aviv University School of Dental Medicine in Israel.
In addition, the study results indicated that almost 80% of patients in the doxepin mouthwash group and 85.5% of patients in the diphenhydramine-lidocaine-antacid mouthwash group experienced clinically important pain improvements, they noted.
"This suggests that a subgroup of patients may have experienced meaningful benefits from the diphenhydramine-lidocaine-antacid mouthwash," they wrote.