
Bleach hurts. Research shows about half of all patients feel some pain when peroxides soak into their teeth. While some studies have found them perfectly safe, others studies suggest they damage exposed dentin, and weaken the cements in fresh restorations. Yet patients still want white teeth.
That's why we sat up in our chair when we received a recent press release from a new company that promises, "the first-ever professional dental health product designed to whiten teeth without sensitivity through a mechanism of 'stain removal' and 'cleaning' rather than traditional bleaching."
The new product is a set of foam-tipped swabs that come with a cleaning solution. We were even more impressed after an interview with the inventor, Martin Giniger, D.M.D., Ph.D., Ms.D., chief science officer for Power Swabs who told us this product would "one day replace toothpaste." Dr. Giniger certainly has the right credentials. In addition to former positions at Discus Dental and the University of Medicine and Dentistry of New Jersey, Dr. Giniger has published extensively in peer-reviewed dental journals.
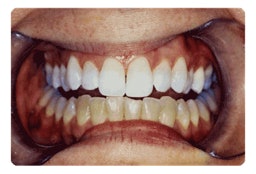 |
| Maxillary teeth treated with Power Swabs vs. untreated mandibular teeth. (Photo courtesy Martin Giniger) |
But other experts in the whitening field are skeptical. There are plenty of nonbleach whitening products already on the market, they say. And there are ways to whiten without harming the patient (see below). But to whiten a tooth inside and out, they've never seen anything better than peroxide.
In an e-mail, Van Haywood, D.M.D., a professor of oral rehabilitation at the Medical College of Georgia, after looking at the Power Swabs Web site, denounced Dr. Giniger's new products as "nothing more than a sham, with no research, and bogus claims that mock the science in bleaching."
Edward J. Swift Jr., D.M.D., an associate editor of the Journal of Esthetic and Restorative Dentistry, and chair of operative dentistry at the University of North Carolina, Chapel Hill, responded more moderately. He's heard of Dr. Giniger's product, and it sounds intriguing. But he wants to see research proving it works.
The claims
Dr. Giniger made a variety of claims for his products, both in his interview with DrBicuspid.com and on two Web sites, one for Power Swabs and another for related products -- a gel, rinse, gloss, and foam, all using the same technology.
In the interview, he explained that his formula cleans teeth by using detergents which actually remove the substance causing stains -- as opposed to peroxides, which change the color of the substance causing a stain. For really white teeth, patients can use his formula first, then bleach afterwards. With less work to do, patients can bleach for a shorter period of time, and thus minimize the potential discomfort.
Using detergents to clean teeth is nothing new, Dr. Giniger admits. Most toothpastes include detergents, as well as abrasives, both of which remove residues of coffee, wine, tobacco, and other substances that stain the surfaces of teeth. What sets Dr. Giniger's formula apart, he says, is that it includes anionic surfactants.
In a nutshell, surfactants (which include soap and detergent) are tadpole-shaped molecules with a hydrophobic (water-hating) tail and a hydrophilic (water-loving) head. The tail bonds to oils in the stain, while the head bonds to water. When the patient brushes, the surfactant's tail pulls the stain off the tooth and into the water.
On this much, all the experts we interviewed agreed. But Dr. Giniger went a step further. Anionic surfactants, says Dr. Giniger, have negatively charged heads that also bind to stains, which are positively charged. The experts we interviewed couldn't confirm or refute the theory. They did point out, though, that anionic surfactants are not unique to Dr. Giniger's formula. As it turns out, sodium lauryl sulfate, present in most toothpastes, is anionic.
Still, Dr. Giniger's web sites boast of other advantages. First, they argue that competing pastes and gels are so thick that they take a long time to penetrate into the tiny nooks and crannies in enamel. By contrast, Dr. Giniger's formula is made of foam. "As the foam collapses back into a thin liquid, it penetrates very rapidly into enamel for FAR FASTER results," one of his Web site says. (The site doesn't explain how another product it advertises, "Highest Quality KIDS SLS-FREE Tooth Cleaning Gel" fits into this theory.)
"Watch us grow from the sidelines as the world changes on my cue."
For perspective on this question, we spoke to Larry Chow, Ph.D., a chemist and chief research scientist at the American Dental Association research foundation. He agreed that a liquid might enter small pores in the surface of enamel more easily than a gel. But, he says, there would be a drawback: One reason we brush our teeth with pastes instead of liquids is that it would be hard to include abrasives in liquids; they would clump together rather than being evenly distributed. Abrasives in toothpaste play an important role, along with detergents, in removing stains.
The research
None of this would matter, if Dr. Giniger could prove that his approach really works better than other products on the market. When asked for supporting studies, he pointed us to two abstracts on his Web site, both for studies apparently conducted by his own company.
One compared his Pearl White Dental Cleaning and Whitening Foam System to "commercially available whitening toothpastes." It concludes that, "After using the Pearl White Foaming System for 4 weeks, it was determined that the test subject’s teeth were 54% cleaner and approximately four shades lighter than the control teeth."
The second compared his Pearl White Dental Cleaning and Whitening Swabs System to "WhiteStrips" -- apparently a reference to Crest Whitestrips. "Immediately after treatment the test group subjects subjective shade improvement was 8.3 shades; the control group improvement was 5.7 shades," this abstract states.
Those studies looked worth reviewing, so we asked Dr. Giniger for the complete text of the two studies, any independent studies on his products, and a list of ingredients for his formula. Dr. Giniger took offense, declining to provide this information, and refused to answer any more questions. "I suggest that you just watch us grow from the sidelines as the world changes on my cue," he wrote.
Advice for patients
So why report on an unproven product? Because it raises a question important to all dentists: what whitening products should you recommend to patients?
Dr. Haywood's position is very clear: carbamide peroxide works best, he argues. He pooh-poohs any concerns about damage to dentin.
Robert W. Gerlach, D.D.S., M.P.H., a research fellow at Procter & Gamble, an adjunct professor at Tufts University, and one of the inventors behind Crest Whitestrips, offered more detail. The detergents and abrasives in whitening toothpastes work OK on residues of coffee, tea, wine, chlorhexidine, and other substances that stain the outsides of teeth (known in the business as "extrinsic" stains.)
But teeth tend to turn yellow or gray with age because of poorly understood chemical reactions. In addition, tetracycline, metabolic disturbances, and fluorosis all changes tooth color from the inside. If you want to attack these "intrinsic" stains, you need something that penetrates enamel and dentin, and nobody has found anything better than peroxide (whether it be hydrogen or carbamide). Peroxide molecules are much smaller than any detergent, so they can get all the way through the tooth.
While putting a little peroxide in a toothpaste might give your detergents and abrasives a boost, soaking teeth for a longer time in higher concentrations works better -- what Dr. Gerlach calls an "intensive" approach. "Make no mistake," says Dr. Gerlach. "Some of these toothpastes can make remarkable changes. But we always find with respect to overall tooth color, the intensive products do better."
Because peroxides thoroughly penetrate the enamel and dentin they can also reach the pulp, which causes sensitivity. But Dr. Gerlach argues that this is a good thing. "It's a little like going to the gym," he says. "No pain, no gain."
That's not to say that patients should bite on a bullet and bleach away. Rather, they should ease off when their teeth start to hurt and find the limit that works for them. Dr. Gerlach advises patients to either skip a treatment day, then start up again, or use the lowest concentration of peroxide available, for the shortest amount of time.
Sensible suggestions. But what about patients who want to refrain from using any kind of bleach at all? We turned to Geza T. Terezhalmy, D.D.S., M.A., a professor of clinical dentistry at the University of Texas, San Antonio, who has been hired by Procter & Gamble, as well as other companies, to test their whitening products.
Like the other experts we contacted, Terezhalmy agrees that only bleach can touch intrinsic stains. As for extrinsic stains, he says look for toothpastes specifically designed to whiten that carry the American Dental Association seal of approval. All these products work, he says, and there's no evidence that one works better than another.
What makes a bigger difference, he says, is the patient's choice of toothbrushes. But which model is best depends on the
- Ask the patient to come to your office in the morning before brushing.
- Stain the patient's plaque with dental disclosing solution and note the results on a plaque index.
- Observe the patient brushing.
- Stain the patient's plaque a second time for a second measurement.
If the results aren't satisfactory, try another brush.
It's old-fashioned -- and a little time consuming -- but it's better than a new fangled product that may or may not pass muster.



















