A man in California has filed a lawsuit against the designers of a device that was marketed to correct dental and facial abnormalities, claiming it is dangerous and left him with pain, jaw problems, tooth damage, and permanent injuries.
Joseph Dimola filed on July 10 in the U.S. District Court for the Southern District of Indiana, Terre Haute Division, a product liability lawsuit against the makers of the Anterior Growth Guidance Appliance (AGGA). Since 2023, several patients have filed lawsuits against the manufacturers and developers of AGGA, gaining the attention of U.S. regulators and other agencies due to claims that the dental device is defectively designed and dangerous.
Dimola is seeking compensatory and punitive damages against Dr. Steve Galella, a dentist in Tennessee and a diplomate of the International Board of Orthodontics, Orthomatrix, which does business as Facial Beauty Institute, Orthologic, and John's Dental Laboratory, alleging they were negligent because they failed to properly warn or train dentists promoting AGGA for adults, according to the lawsuit.
Though Dimola had the device removed due to TMJ and bite problems and more, he continues to have facial and jaw tension and migraines, according to the suit.
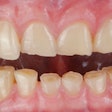
In December 2020, Dimola had the AGGA device installed in his mouth. The device, which is supposed to remodel or expand the jaw of an adult without surgery, consists of a metal wire insert that is placed between the teeth. In actuality, the suit claims that the device pushes the upper teeth out of place, leading to gum damage, tooth loss, nerve problems, disfigurement, and other complications that require corrective procedures.
Despite having the device removed in May 2021, Dimola alleges that he continues to experience significant and permanent injuries and damage, including neck pain and tightness, body discomfort, facial asymmetry and disfigurement, TMJ pain, sleep apnea, tooth sensitivity, an uneven bite, and difficulty eating and opening his mouth.
"AGGA was unproven, was not supported by scientific or medical knowledge, was not reasonably safe, was unreasonably dangerous, was not efficacious, and presented a risk of serious and permanent injury to consumers," according to the lawsuit.