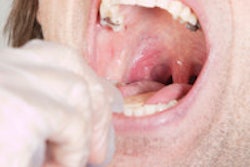
A U.S. government-backed task force issued a statement this week saying that there is not enough published evidence to recommend for or against screening for oral cancer by primary care professionals.
Evidence is lacking on whether screening can accurately detect oral cancer and if earlier treatment of cancers found during those tests improves long-term health, according to the U.S. Preventive Services Task Force (USPSTF).
Their draft recommendation statement applies to people who do not have any signs or symptoms of oral cancer and is meant for primary care professionals screening for oral cancer. It is not a recommendation about the practices of dentists and oral health professionals, the panel noted.
The task force -- an independent volunteer panel of national experts in prevention and evidence-based medicine -- reviewed the current literature and found:
- Inadequate evidence that the oral screening examination accurately detects oral cancer
- Inadequate evidence that screening for oral cancer and treatment of screen-detected oral cancer improves morbidity or mortality
- Inadequate evidence on the harms of screening; no study reported on harms from the screening test or from false-positive or false-negative test results
Seven studies (n = 49,120) examined the performance characteristics of the oral screening examination. These studies were generally conducted in settings with an increased incidence of and mortality from oral cancer (India, Taiwan) compared with U.S. rates, the panel reported. The studies also had considerable heterogeneity and demonstrated great variation in test performance characteristics. Across the seven studies, sensitivity for oral cancer or potentially malignant disorders ranged from 18% to 94.3% and specificity from 54% to 99.9%. The positive predictive value ranged from 17% to 86.6% and the negative predictive value from 73% to 99.3%.
Two studies in the U.K. looked at oral examinations performed by general dentists among older adults (age 40 years or older) at increased risk because of alcohol and tobacco use and a mixed sample with unknown risk factors. The dental examination in the high-risk sample (n = 2,027) showed a sensitivity of 74%, a specificity of 99%, and a positive predictive value of 67%, while the study of patients with unknown risk factors found a sensitivity of 71%, a specificity of 99%, and a positive predictive value of 86%.
Although the patients in the U.K. study may be similar to the U.S. population, the results of these studies were limited by an imperfect reference standard, by combining the detection of potentially malignant disorders with oral cancer and an unclear delineation of high-risk status, according to the USPSTF.
“The evidence shows that it is difficult to detect oral cancer and that the evidence is not clear whether oral cancer screening improves long-term health outcomes among the general adult population or among high-risk groups,” stated task force member Jessica Herzstein, MD, MPH, said in a news release. “We need more high-quality research on whether screening tests can accurately detect oral cancer and if screening adults for oral cancer in primary care settings improves health outcomes.”
But Brian Hill, executive director of the Oral Cancer Foundation, took issue with the task force's recommendations.
"I put no weight on what the U.S. Preventive Services Task Force has to say about this since their determination was based on the evidence that no peer-review studies have been done to show that oral cancer screening has any impact on long-term outcomes," he stated. "Lack of published data showing benefit or harm is only evidence of a lack of published data, not evidence of a negative finding."
In addition, he said, "We know from the best database of disease rates and outcomes in the U.S., the SEER database [Surveillance, Epidemiology, and End Results] -- which is how we track incidence, causes, and outcomes in the U.S. -- that stage I oral cancer patients have better outcomes and stage IV patients have poorer outcomes. And stage I people have, besides longer lives, far fewer treatment-related morbidity issues to get to a point of no disease."
The new recommendations, currently in draft form, are available for public comment April 9 through May 6.