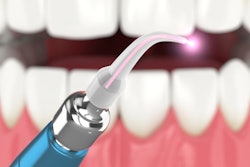
The U.S. Food and Drug Administration (FDA) has given 510(k) clearance to Convergent Dental's all-tissue dental laser Solea, expanding its use as a caries prevention tool.
The 9.3 µm carbon dioxide laser can be used to aid in the reduction of mineral loss in dental enamel. Solea is designed to help protect teeth against the effects of acid and resulting tooth decay development.
Starting in October, the laser will be available commercially on a limited basis. In 2023, it will be fully available. Convergent Dental also plans to apply for clearance with Health Canada.