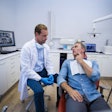
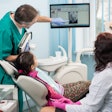
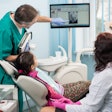
The U.S. Food and Drug Administration (FDA) has given de novo approval to DentalMonitoring for its artificial intelligence-powered software that remotely monitors orthodontic treatment.
The software, which is indicated for patients over the age 6 and solely for permanent teeth, uses image-processing algorithms to analyze pictures of the oral cavity. Using the company app, a smartphone, and the manufacturer's proprietary hardware products, scans are taken for analysis. Furthermore, the software aims to offer additional insight into treatment progress while patients receive automated notifications and in-app communication with practice staff.
With the FDA's approval, DentalMonitoring will introduce the SmartSTL feature, which allows clinicians to request an updated stereolithography (STL) file via the company's dashboard, to orthodontists in North America.