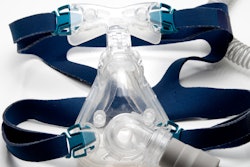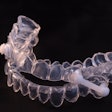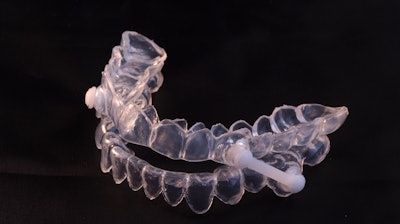
The U.S. Food and Drug Administration (FDA) has cleared AIO Breathe, a device used to treat mild to moderate obstructive sleep apnea (OSA) from biomedical firm Aiomega.
The new mandibular repositioning device is designed to increase a patient’s airway during sleep, reducing the tendency to snore and alleviating signs of OSA. The device features protrusive flanges that engage with the right and left vertical sides, optimally repositioning the jaw. Additionally, mandibular plateaus direct the mandible downward, thereby opening the anterior airway. Mandibular advancement is maintained whether the individual's mouth is open or closed.