CHICAGO - In a series of press conferences at the Chicago Dental Society Midwinter Meeting, several new products were introduced that their makers claim will significantly impact clinical, preventive, and cosmetic applications in dentistry.
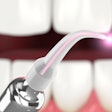
Biolase Technology announced the latest addition to its soft-tissue laser product line: the iLase. Dubbed the first "personal laser" for dental professionals, the device is designed to make laser technology available to every dentist and hygienist in a practice, the company said.
The pen-sized, diode-laser device is a completely self-contained, battery-powered handpiece that requires no power cord or foot pedal to operate. It weighs 3.5 ounces (less than 100 grams) and is approximately 7 inches long (without tip). Tip lengths vary from 4 mm to 14 mm. The system features 10 preset soft-tissue and hygiene procedures, including gingivectomy, troughing for crown procedures and sulcular debridement, and cleaning between gums and teeth for treating periodontal disease.
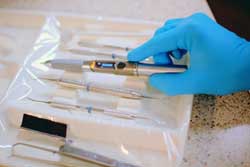 |
|
The Biolase iLase handheld wireless soft-tissue laser. Image courtesy of Biolase Technology. |
"A clinician can easily carry all the components of the laser in one hand and you don't have to plug it into a power outlet," said CEO David Mulder. "That's why we call it the first 'personal' laser. There is nothing on the dental laser market like it, and we expect the device to significantly improve workflow in the dental suite, by making it easier than ever for any dentist and hygienist to bring laser technology to any patient."
Although the iLase is still awaiting FDA 510(k) clearance -- the company is uncertain when exactly this will happen, although they have filed for it -- it does have CE Mark approval and is available for purchase in Europe. Biolase said that once the iLase becomes available in the U.S., it will be "competitively priced," selling for less than its ezlase, which is being offered at the Chicago Midwinter Meeting for $8,000.
The company said that while it has conducted clinical research with the iLase and that the results of that research have been submitted it to the FDA, no papers have been published involving this research to date.
OralDNA and HPV
OralDNA Labs is expanding its portfolio of salivary diagnostics products with the OraRisk HPV test, a noninvasive screening tool to identify oral human papillomavirus (HPV) and provide an earlier screening method for related cancers.
Oral HPV has been shown to be a risk factor for oral, head, and neck cancers. According to the Centers for Disease Control and Prevention, 20 million people in the U.S. currently have HPV. High-risk types of HPV that persist present an increased risk for oral, head, and neck cancers, according to the company.
In fact, while tobacco and alcohol use have traditionally been considered to be the primary risk factors for squamous cell carcinoma of the head and neck (SCCHN), a new high-risk profile for SCCHN has emerged, said Ronald McGlennen, M.D.
"Recent research studies have identified several high-risk strains of the HPV, especially the variant known as HPV-16, as potential etiologic agents in the development of SCCHN," he said. "The availability of the OraRisk HPV test marks an important and timely advance in oral diagnostics."
The OraRisk test will provide the dental clinician with the ability to establish risk for HPV-related cancers of the oral, head, and neck regions, and determine appropriate referral and monitoring conditions, according to the company.
"Oral HPV is a silent, serious infection that can now be detected and closely monitors by the dental professionals," said Thomas Nabors, D.D.S., OralDNA Labs' chief dental officer. "Specifically, the laboratory report derived from the OraRisk HPV salivary diagnostic test helps dental professionals identify the specific types of oral HPV present, as well as the associated risk profile for each type of HPV variant detected in the patient's oral cavity."
The test costs $199 with a $70 associated lab fee, the company said.
OralDNA already markets two related salivary diagnostic products: MyPerioPath and My PerioID. MyPerioPath detects the presence and quantity of specific bacteria associated with periodontal disease; MyPerioID assesses an individual's genetic risk for periodontal disease.
Spray-on whitener
Wow Oral Care launched a more potent version of its Spraywhite 90 teeth-whitening system. While the Spraywhite 90 was already available to consumers online, the Spraywhite Pro will be available exclusively through dentists and is designed to dramatically reduce the time it takes to achieve the desired whitening effect.
"Since this is a stronger formula we wanted to make sure that dentists are involved with this product," Greg Vigoren, D.D.S., vice president of research and development, told DrBicuspid.com.
The Spraywhite system involves a two-second spray application on clenched teeth, followed by swishing for 30-90 seconds. One of the unique properties of the product is the ability to vary the time and concentration to accommodate individual response variations to whitening, according to the company. This delivery system allows for a short exposure time with a small volume of spray that can be diluted rapidly with saliva by swishing.
The Spraywhite Pro is designed to work in conjunction with the Wow Powder Oral Rinse, a biofilm stripper and bactericidal agent "masquerading" as a breath and taste freshener, the company said. The ability of the oral power rinse to protect the mouth from any adverse pH changes can drive tooth sensitivity issues to very low and manageable levels, the company said. In addition, as the second step in the Spraywhite system, the oral rinse leaves a much fresher aftertaste, according to the company.
Used together, these products remove stains and also help to prevent gum irritation, Wow said.
"Spraywhite Pro is the first of the next generation of whitening products with genuine oral hygiene benefits," said Michael Arnold, founder of Carson Labs and inventor of the Wow whitening system.
Wow selected a number of dentists to conduct patient trials on Spraywhite Pro last August. Participating dentists reported benefits such as reduced chair time and staff time required to attend to patients, as well as a lower cost compared to other laser/light-activated teeth-whitening processes, the company said.
Most in-office whitening procedures take up to two hours of staff time, while this system takes five minutes from explanation to application, according to Dr. Vigoren.
"By drastically reducing chair time, you can increase the number of patients who receive whitening in your practice," he said. The product can also be used as a whitening maintenance regimen for patients that invest in laser or other whitening methodologies, he said.
Dentists can provide Spraywhite Pro to their patients by offering a sample kit with a referral code. The patient then goes to the company Web site and plugs in the referral code, which will allow them to buy Spraywhite Pro at a discount while giving the referring dentist a credit. Dentists can also buy the product in bulk and sell it directly to their patients. Using the referral code approach lowers the price of the kit from $299 to $199.
Copyright © 2010 DrBicuspid.com