The first lawsuit against Merck regarding the relationship between the drug Fosamax and the development of osteonecrosis of the jaw (ONJ) may have ended in a mistrial, but with hundreds of similar lawsuits still pending, the question remains: Does the use of Fosamax or other bone-enhancing drugs lead to ONJ?
The answer has important ramifications for dental care in certain patient populations.
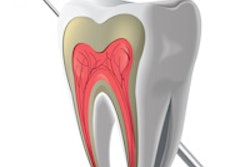
The problem lies with the main ingredient in Fosamax and other drugs that build bone mass and stem bone loss -- bisphosphonates.
Bisphosphonate-related osteonecrosis of the jaw (BRONJ) is a relatively new condition triggered primarily by increased use of bisphosphonate-based drugs.
It is defined by masses of exposed bone in the mouth where the gums have completely fallen away from the bone and the bone is dead, explained Marshall Wade, D.D.S., an oral and maxillofacial surgeon from Minnesota who has written and lectured on the topic, in his seminar "Osteonecrosis of the Jaw: Cutting Through the Confusion" at the recent California Dental Association (CDA) fall session in San Francisco.
According to Dr. Wade, although BRONJ is a multifaceted problem, it can be pinpointed:
- The patient must have current or previous treatment with a bisphosphonate medication.
- The patient must have exposed bone in the maxillofacial region for more than eight weeks.
- The patient should have no history of history radiation exposure to the area.
There has been a lot of overreaction to the condition, Dr. Wade said. The initial literature suggested that there was very little dentists could do once the bone was exposed, because the half-life of the drug is 10 years and it stays in the bone for 20 years. But we now know that is not entirely true, he said.
"We are watching the evolution of an entirely new disease, and everything we know or discuss today could change tomorrow," he added.
— Marshall Wade, D.D.S.
BRONJ case studies
Dr. Wade illustrated how dentists can manage the condition by sharing case studies of patients who took bisphosphonate medication to build bone (as opposed to being part of a cancer treatment). In each instance, he asked the patients four key questions:
- Why are you taking the medication?
- How long have you been taking it?
- Do you know the dosage you take?
- What other medications do you take?
Palatal torus
The first case Dr. Wade presented was that of a 75-year-old white woman who had been taking alendronate (Fosamax) for more than five years.
After she broke her hip, the doctor was concerned that it might happen again, and a scan showed that she had osteoporosis. Also, she showed evidence of spinal compression with deformity.
She came in for a palatal torus removal, and she also had retained dental roots that required extraction in preparation for complete upper denture. Her mucosa, floor of mouth, and posterior pharynx were clear.
Dr. Wade explained that he did not want to remove the torus because of the risk for BRONJ. He was even apprehensive about taking out the teeth but had no choice in the matter because of multiple problems.
Another treatment consideration was whether he could take the patient off her bisphosphonate medication for the dental treatment.
In this case, he decided against it because the patient was a true osteoporotic and had already suffered two fractures. Her physician also recommended that she should stay on her medication.
"I think it's common courtesy for us not to tell patients to discontinue their medication, but rather to communicate with the prescribing physician to see if that patient can come off the medication," Dr. Wade said.
A 2006 study compared the effects of discontinuing alendronate treatment after five years versus continuing for 10 years in 1,099 postmenopausal women. One group took alendronate for five years and a second group took it for 10 years (Journal of the American Medical Association, December 27, 2006, Vol. 296:24, pp. 2927-2938).
The researchers found that women who discontinued alendronate after five years showed a moderate decline in bone mineral density and a gradual rise in biochemical markers but no higher fracture risk.
The one group that did demonstrate an increased fracture risk consisted of patients with previous spinal compression or fracture, Dr. Wade noted. And that is why it was important to keep this particular patient on her medication, he said.
As for her dental needs, the restorative dentist made her denture by using a soft reline material over the torus. Also, the dental roots were extracted and she was scheduled for follow-up. She tolerated the treatment well and did not develop any osteonecrotic areas.
Polymyalgia rheumatica
The second patient was a 55-year-old white woman with polymyalgia rheumatica who came in for an extraction. Her doctor told her that her bones could become brittle from other medication she had been taking.
She had been on alendronate for three years, starting at 10 mg per day and later increasing to 20 mg per day. She also took prednisone, which shortens the time period in which BRONJ can develop. Other medications included an antidepressant, a blood pressure pill, and the occasional Vicodin.
In this case, Dr. Wade discussed the possibility of using a serum C-terminal telopeptide (CTx) test, which initial data suggested could be useful in evaluating healing capability by assessing osteoclastic function.
Researchers of a 2007 study also found favorable results, concluding that "the morning fasting serum C-terminal telopeptide bone suppression marker is a useful tool for the clinician to assess risks and guide treatment decisions" (Journal of Oral and Maxillofacial Surgery, December 2007, Vol. 65:12, pp. 2397-2410).
However, much like some of the studies before it, this one had its limitations, which cast doubts on the accuracy of the serum CTx test results.
An American Society for Bone and Mineral Research (ASBMR) task force responded to the 2007 article by stating that it is "not appropriate to base clinical recommendations on an uncontrolled study of 30 patients." The ASBMR response article also stated that the 2007 study "may cause dental professionals to adopt a set of behaviors that is not only based on inadequate evidence but may deprive patients of much needed therapy for their osteoporosis" (J Oral Maxillofac Surg, June 2008, Vol. 66:6, pp. 1320-1321).
Dr. Wade agreed. "My recommendation to you would be not to hang your hat on a CTx value," he said.
If the tooth is painful and at risk for infection, perform an extraction, he said. The risk of infection in a patient on steroids far outweighs the risk of developing BRONJ. However, it is still appropriate to contact the patient's physician to discuss a drug holiday to aid in healing. It is also essential to advise the patient of possible risk and obtain signed consent.
However, if the teeth are not painful or at risk for infection, the American Association of Oral and Maxillofacial Surgeons (AAOMS) recommends that the bisphosphonate medication should be discontinued three months prior to extraction until osseous healing occurs (J Oral Maxillofac Surg, May 2009, Vol. 67:5, Supplement).
"I have not seen anything in the literature that would indicate that a standard three-month drug holiday would make any difference," Dr. Wade said. "While you could do that and you would be within the standard of care, I don't think it would necessarily make a great deal of difference."
Dr. Wade also recommends antibiotics for these patients. He suggests putting them on the antibiotics before the operation and for a week after the surgery.
Implant: Yes or no?
The third patient was a 48-year-old white woman requesting an implant. She had received one injection of zoledronic acid (Reclast) that year, and she had taken the oral medication for a year before recently switching to the injectable form. Her mother had brittle bones, and because she was at a similar risk, she was taking the medication to improve bone strength.
Patients are at risk for BRONJ after long-term use of oral bisphosphonate medicine, Dr. Wade said. "I consider anything over three years to be long-term bisphosphonate use," he added.
The complication in this case lies in the fact that experience with injectable bisphosphonates -- when they are used in a dosage appropriate for osteoporosis -- is limited. And given the one-year use of oral bisphosphonate, the risk of developing ONJ appears very small. However, if BRONJ is related to cumulative dose, which may be true, there is no way to be sure in this particular case.
There is some very conflicting data on this topic, Dr. Wade said.
A 2008 study looked at 600 patients who had implants placed and were also on bisphosphonate medication. A total of 29 implants failed, of which eight were early and 21 were late failures. The failure rate was still 1% or less (J Oral Maxillofac Surg, August 2008, Vol. 66:8, Supplement, pp. 46-47).
However, this was a retrospective study obtaining answers to a survey with no record of when or how much bisphosphonate the patient was taking. Also, BRONJ was not mentioned in the article, Dr. Wade explained.
Another study concluded that "on the basis of two controlled clinical studies, oral bisphosphonate usage was not associated with occurrence of ONJ" (International Journal of Oral and Maxillofacial Implants, May/June 2006, Vol. 21:3, pp. 349-353).
However, in this study the subjects started taking alendronate when the implants were placed, and the study was concluded before year three.
A more useful study looked at 1,319 patients from Montefiore Hospital who had implants placed between 1998 and 2006. Researchers sent out a survey about bisphosphonate therapy to all 1,319 patients. Responses were received from 458 patients, of whom 115 reported that they had taken oral bisphosphonates (J Oral Maxillofac Surg, February 2008, Vol. 66:2, pp. 223-230).
The researchers found no evidence of BRONJ in any of the patients evaluated in the clinic. Of the 468 implants, all but two integrated fully and meet criteria for establishing implant success.
"Implant surgery on patients receiving bisphosphonate therapy did not result in bisphosphonate-associated osteonecrosis of the jaw," the authors concluded. "Nevertheless, sufficient evidence exists to suggest that all patients undergoing implant placement should be questioned about bisphosphonate therapy including the drug taken, the dosage, and length of treatment prior to surgery."
The researchers also suggested additional testing and alternate treatment options should be considered for patients with a history of oral bisphosphonate treatment exceeding three years and those having concomitant treatment with prednisone.
"I am not placing implants in patients who have been on their bisphosphonate medicine for longer than three years," Dr. Wade said.
And as for patients who have been on them for less time, he has a long conversation with them about the potential for delayed healing, osteonecrosis, and other risks.
"I am very conservative," he said. "If any of you have had implants fail, you know it's not fun. And on top of that to have the risk of BRONJ -- I am just not willing to take that risk."
For the full presentation and more information on BRONJ, visit Dr. Wade's Web site.
Copyright © 2009 DrBicuspid.com